Learn About Librium Being Discontinued in 2021
Librium was the first benzodiazepine marketed. Librium was FDA-approved in 1960 and became the prototype for the numerous benzodiazepine compounds such as Valium that followed. In 2021 the brand name Librium was discontinued presumably after patent expiration, though we are still looking for confirmation and any related details. The generic equivalent benzodiazepine is called chlordiazepoxide and is still prescribed in certain instances.1
Librium heralded a new age of benzodiazepine tranquilizers, replacing earlier barbiturates that had gained great concern over safety issues. Initially, benzodiazepines were promoted as less addictive and causing less cognitive impairment than their predecessors. Soon after the release of Librium and the offshoots, however, it became increasingly clear that benzodiazepine drugs can also put a person at high risk for dependency and addiction. Because of this, we see that Librium is not free of side effects as was originally hoped.
These and other topics, including Librium withdrawal and other frequently asked questions about Librium are further outlined below.
What Is Librium (chlordiazepoxide) Used For?
Librium is approved for short-term anxiety relief. The drug label stipulates it should not be taken for more than 4 months.1 However, other recommendations caution us that benzodiazepines be taken for several weeks at most. The guidelines proposed by Ashton recommend using benzodiazepines for a few days to a few weeks at maximum.14
Off-label uses for Librium include acute alcohol withdrawal and as a bridge medication for cessation from some other sedatives. It is also used as a pre-surgical sedative to reduce apprehension and fear. These would be short-term uses and would be in accord with the recommendations noted above.
Over the past 50 years, Librium has been used off-label to treat various disorders such as chemotherapy-associated nausea and psychogenic catatonia. According to research by Benjelloun et al., catatonia and NMS are thought to be possibly related disorders, and the authors also suggest these conditions may be a result of a “dopamine deficiency.” Because GABAergic drugs such as benzodiazepines are thought to influence dopamine receptor sin various ways, this may explain at least in part, why some cases of NMS disorders are caused by neuroleptic (extremely sedating) drugs and why other cases may receive the same drugs therapeutically to alleviate these types of disorders. These cases tell us that there is much yet to be discovered about how to safely use benzodiazepines and other neuroleptic drugs as valid therapeutics.11-13
The drug is not FDA recommended for children under the age of 6.1
The use of Librium during pregnancy has not been studied.18
Librium (chlordiazepoxide) Alternative Names and Slang
The generic drug name for Librium is chlordiazepoxide.
Librium and other benzodiazepines have developed a fairly common presence on the street, possibly due to their perceived usefulness in easing withdrawals from other street drugs, producing a euphoric and relaxation effect. Benzodiazepines have acquired many nicknames as street drugs, such as benzos, heavenly blues, bars, goofballs, nomies, tranx, sleepers, valley girls, “blue bombs,” for example.
Librium (chlordiazepoxide) Side Effects
Librium is a potent CNS depressant, producing a sedating, calming effect in human subjects. In clinical trials using monkeys, hostile monkeys were made tame with low doses of Librium, without sedative effects. The drug reportedly reduced aggression and fear in these monkey trials. The same experiment yielded similar results when performed on mice.1,15
If mild to moderate adverse effects do emerge, your physician may suggest reducing the dosage. For life-threatening adverse effects, emergency treatment may be required, including arranging immediate transport, by ambulance if needed, to the nearest hospital.
Librium side effects include:
- Sedation
- Mania, hyperexcitability, nervousness
- Hallucination
- Dizziness
- Fatigue, weakness
- Depression
- Confusion
- Memory impairment
- Nausea, vomiting
- Slurred speech
- Hyper-salivation
- Dry mouth
- Ataxia — loss of balance, trouble walking, speech difficulties
- Headache
- Rash, skin eruptions
- Constipation
- Swelling
- Depression
- Loss of libido
- Menstrual issues
- Hypotension
- Weight gain
- Blood disorders
- Slowed respiration
- Renal dysfunction
Coming Off Librium and Other Drugs Safely
The FDA recommends not to abruptly stop taking Librium, but to gradually reduce the dosage over a period of time to avoid these harsh adverse effects. These recommendations apply to virtually all drugs. Clinical trials published in the Journal of Psychopharmacologia show that abrupt Librium withdrawal is not advised because of patient safety issues.16
The half-life of the drug has been shown to vary anywhere from 7 to 52 hours, which is relatively long for a benzodiazepine. Data cited from Heather Ashton’s work and others, notes the active metabolite in the drug has a half-life of up to 200 hours.29 The dosage strength, how long it has been taken, and the presence of liver impairment are factors that influence the half-life elimination rates, as related to the accumulation and elimination rates of metabolites in the system.17
Librium should not be stopped cold turkey unless there is a sound medical reason for doing so. In the case of life-threatening adverse reactions, Librium cessation would best be done in a hospital setting. Abrupt discontinuation could result in seizures and other health issues, as noted above. In most other cases, protracted (harsh, long-lasting) withdrawals may result from abrupt cessation, possibly due to neuron damage.14
The most reasonable approach to stopping Librium is to do a gentle and gradual reduction with medical supervision. Sometimes a residential rehabilitation setting can be warranted as Librium withdrawal can often be painful, especially when adjunct therapies are not provided. The advantages of choosing a facility where the staff members and caregivers are experienced with Librium withdrawal, and where ample onboard therapies are available, cannot be overstated.
 Withdrawal symptoms can last a few weeks to significantly longer. Some patients report protracted withdrawal symptoms lasting even years, especially without proper treatment.
Withdrawal symptoms can last a few weeks to significantly longer. Some patients report protracted withdrawal symptoms lasting even years, especially without proper treatment.
Patients reporting protracted withdrawal may express feelings of anxiety and other symptoms similar to those of early withdrawal.
The literature of Dr. Heather Ashton theorizes that this might be due to damaged receptors.14
Alternative to Meds Center designs holistic withdrawal programs based on therapies that will promote neurotransmitter rehabilitation as a fundamental for authentic recovery.
Removal of Neurotoxins
Another suspected etiology (cause of illness or symptoms of mental illness) that we have seen clinically is the ongoing effects of a body burden of neurotoxic poisoning. It is common for these individuals to be misunderstood, and subjected to doubt from family members, friends, and even medical providers. Unfortunately, they tend to be further pathologized and often disregarded. It is likely that mainstream viewpoints need to shift and take a deeper look at what these damaged people are truly experiencing and offer them hope and guidance, rather than abandonment. Holistic, non-invasive neurotoxin removal is a fundamental pillar of Librium withdrawal at Alternative to Meds Center. Please take a moment to review the protocols used at Alternative to Meds Center. Neurotoxins come in many forms, from pesticides to cosmetics and foods, and we have all been subjected to these exposures, and potentially suffer from symptoms of toxic exposures, that are sometimes misdiagnosed as puzzling mental disorders.21
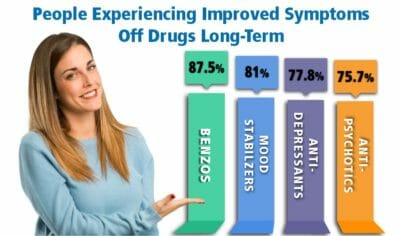
 Withdrawal symptoms can last a few weeks to significantly longer. Some patients report
Withdrawal symptoms can last a few weeks to significantly longer. Some patients report  There are hundreds of known medications that can interact with Librium and may cause adverse effects as a result.1,26 Some of the medications to avoid if you are taking Librium include:
There are hundreds of known medications that can interact with Librium and may cause adverse effects as a result.1,26 Some of the medications to avoid if you are taking Librium include:
 Alternative to Meds Center is an inpatient treatment center that specializes in healthy
Alternative to Meds Center is an inpatient treatment center that specializes in healthy  Witnessing a loved one struggling through such challenges can impose significant and even emotionally painful challenges on the family. Deciding on inpatient care can ease the situation both for the family members and for their loved ones, allowing everyone to focus more fully on recovery.
Witnessing a loved one struggling through such challenges can impose significant and even emotionally painful challenges on the family. Deciding on inpatient care can ease the situation both for the family members and for their loved ones, allowing everyone to focus more fully on recovery.







